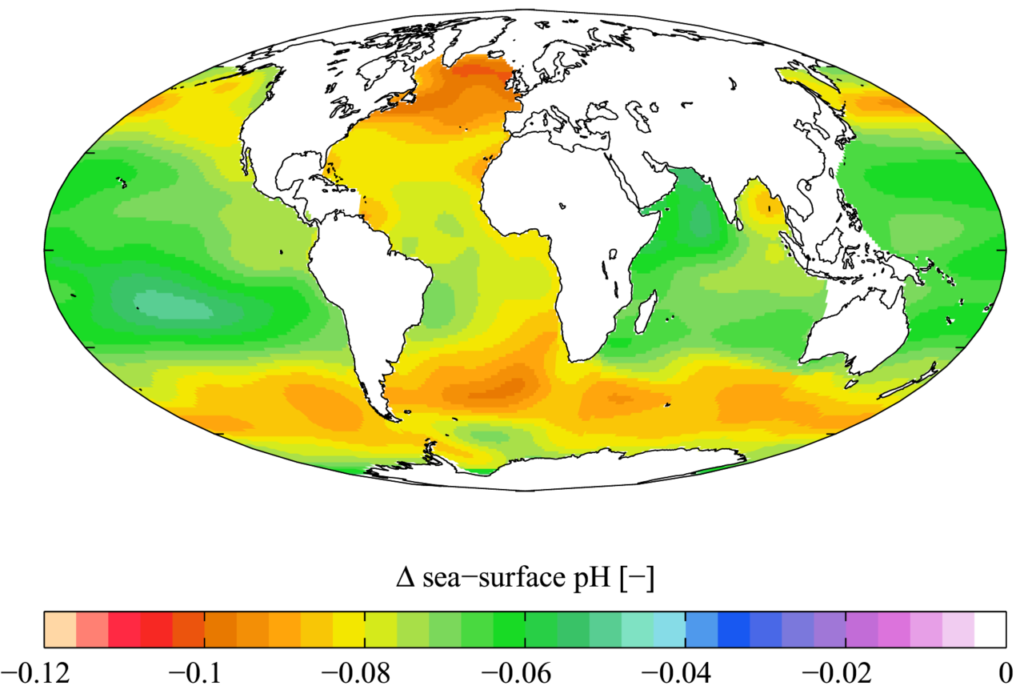
Oceans absorb approximately 22 tons of carbon dioxide daily, and the pH of the world’s oceans are expected to decrease from pH 8.1 to pH 7.8 by the end of this century. The decline in pH is detrimental to many marine organisms, especially those with shells such as zooplankton and coccolithophores. These organisms are important because of their position in the food web. [2]
Video summary: When carbon dioxide in the atmosphere dissolves in sea water, carbonic acid is formed. Carbonic acid negatively affects the ability of marine organisms such as clams, snails, and corals to form their calcium carbonate shells. Since many of these organisms are the main food sources of larger organisms, their disappearance could have major effects on the food web.